Energie Department
We sell artificial oil production equipment that can produce oil (n-alkanes) from carbon dioxide gas and water.
Petroleum is chemically synthesized efficiently at room temperature and pressure, and sulfur oxides (SOX) and nitrogen oxides (NOX) are not emitted after combustion.
There are various types of oil that can be manufactured, including diesel oil and heavy oil. Aiming for a decarbonized society that is friendly to the health of the earth and people, this is a dream renewable fuel production device that enables the production of renewable energy through carbon dioxide fixation.
*Composition during diesel oil production C: approx. 86%, H: approx. 14%, N: 0%, O: 0% (elemental analysis results)
Overview of artificial fuel production equipment
POINT 01 :Oil can be produced using carbon dioxide gas and water
Petroleum is produced by mixing petroleum seed with activated water (radical water) made from ultrafine carbon dioxide (CO2) bubbles and a special photocatalyst. It is abiotic oil production and the material is virtually never depleted.
POINT 02 :Total calorific value is almost the same as commercial oil
The artificial fuel (new oil) produced by this device and the commercially available oil (original oil) used as seed have the same calorific value. New oil 46,010J/g Original oil 45,990J/g *Measurement results according to Japanese Industrial Standard JIS K2279.
POINT 03 :Low running costs
Fuel is produced at room temperature and pressure. It doesn’t require huge equipment either. Therefore, we have achieved low running costs.
POINT 04 :The size of the device is equivalent to 2.5 20ft containers
When you hear of equipment for producing oil, you may imagine a huge facility. This artificial fuel production equipment compactly integrates the performance of 2.5 20ft containers, achieving extremely high productivity.
*20ft container external dimensions (ISO standard): 6,058×2,438×2,591mm
POINT 05 :Can handle light oil, heavy oil, and kerosene
In addition to water and carbon dioxide, the production of artificial fuel requires oil (original oil) as a seed. If the base oil is light oil, it can be made into artificial light oil, and if the base oil is heavy oil, it can be made into artificial heavy oil. The same goes for tetradecane. Furthermore, artificial light oil can be made from artificial light oil. In other words, new oil is synthesized using the used oil as a template.
POINT 06 :Do not emit SOX after combustion
The artificial fuel produced by this device does not contain sulfur (S) or nitrogen (N). Therefore, even when burned, sulfur oxides (SOX), which cause asthma, acid rain, and photochemical smog, are not generated. Nitrogen oxide NOX is also not generated except for NOX derived from N2 in the air.
POINT 07 :Fuel efficiency increases up to 1.4 times with artificial light oil
The artificial light oil produced by this equipment complies with Japanese Industrial Standards (JIS). Using this product, we have succeeded in increasing the fuel efficiency of a car’s engine by 1.15 to 1.4 times* and extending its driving distance. What’s more, soot is drastically reduced. Not only is the exhaust gas cleaner, but the engine noise is also quieter. Knocking almost no longer occurs.
*The numbers are just examples.
POINT 08 :Significant contribution to preventing global warming
One of the materials for artificial fuel is carbon dioxide (CO2). In other words, manufacturing consumes carbon dioxide gas. This makes emissions trading*1 advantageous at both the corporate and national levels. In addition, we can expect to achieve ultimate carbon neutrality*2 (carbon neutrality), contributing to a decarbonized society.
*1 Emissions trading: A system in which each country or company has a greenhouse gas emission allowance, and the surplus emissions allowance can be traded with the company or country that has exceeded the allowance.
*2 Carbon neutrality: The concept that the amount of CO2 emitted and absorbed during human activities is the same.
POINT 09 :Improving business competitiveness
Artificial fuels can reduce fuel costs for trucks and ships, and if used for thermal power generation, we can expect to see lower electricity bills in the future. This will improve the base of business competitiveness and, by extension, international competitiveness.
How artificial fuel is made
STEP 01
Supply nanobubbles (ultrafine bubbles) of carbon dioxide (CO2) to water. Nanobubbles are air bubbles on the order of nanometers. These bubbles do not rise to the surface of the water, but remain in the water.
STEP 02
The water from Step 1 is passed through a special photocatalyst column and irradiated with ultraviolet rays while circulating the water in the presence of trace amounts of oxygen.
Then oxygen turns into ozone. When ozone reacts with a photocatalyst, active oxygen such as hydroxyl radicals are generated. This active oxygen reduces carbon dioxide gas to carbon monoxide. 2CO2 ⇒ 2CO + O2 (Reaction 1) Photocatalyst decomposes water and produces hydrogen and oxygen. 2H2O ⇒ 2H2 + O2 (Reaction 2) Reactions 1 and 2 result in CO2 + H2O ⇒ CO + H2 + O2 (Reaction 3), generating carbon monoxide, hydrogen, and activated water in a radical state that easily causes chemical reactions.
STEP 03
Mix activated water and hydrocarbons (seed oil). Then, it emulsifies and becomes cloudy.

STEP 04
Additionally, carbon dioxide gas from the air is also absorbed. Next, the carbon monoxide and hydrogen produced in step 3 undergo a chain reaction through radical polymerization, producing hydrocarbons (petroleum). nCO + (2n+1) H2 ⇒ CnH2n+2 + nH2O (Reaction 4) Therefore, nCO2 + (n+1) H2 ⇒ CnH2n+2 + nO2 (Reaction 5) When left to stand, it separates into an aqueous layer and oil layer.

Water decreased by 10% and hydrocarbons (oil) increased by approximately 10%
Comparison of commercially available diesel oil and artificial diesel oil
Comparison of components The component distribution by carbon number is as follows.
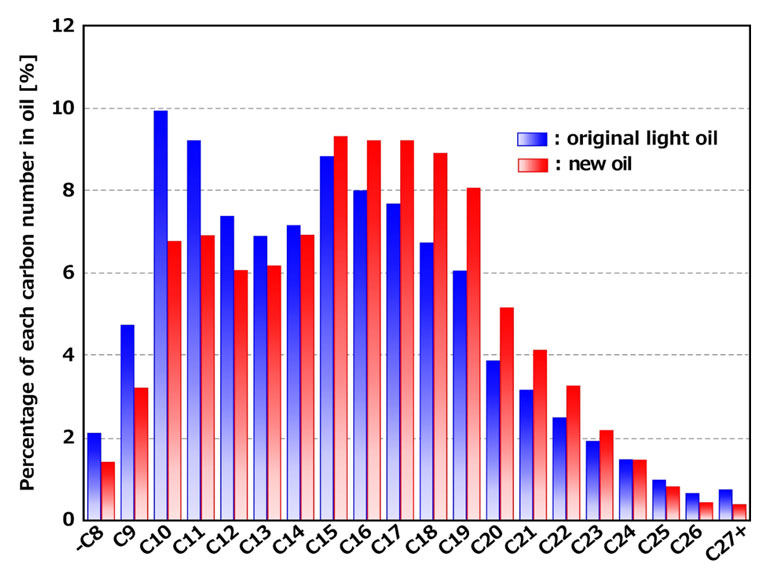
| Carbon nomber in oil | Original oil | New oil |
| C8 or less | 2.11 | 1.41 |
|---|---|---|
| C9 | 4.75 | 3.20 |
| C10 | 9.93 | 6.78 |
| C11 | 9.23 | 6.92 |
| C12 | 7.38 | 6.06 |
| C13 | 6.90 | 6.18 |
| C14 | 7.16 | 6.93 |
| C15 | 8.84 | 9.32 |
| C16 | 7.99 | 9.24 |
| C17 | 7.69 | 9.21 |
| C18 | 6.72 | 8.91 |
| C19 | 6.06 | 8.06 |
| C20 | 3.86 | 5.16 |
| C21 | 3.16 | 4.13 |
| C22 | 2.49 | 3.26 |
| C23 | 1.92 | 2.19 |
| C24 | 1.47 | 1.46 |
| C25 | 0.97 | 0.81 |
| C26 | 0.65 | 0.43 |
| C27 or more | 0.73 | 0.37 |
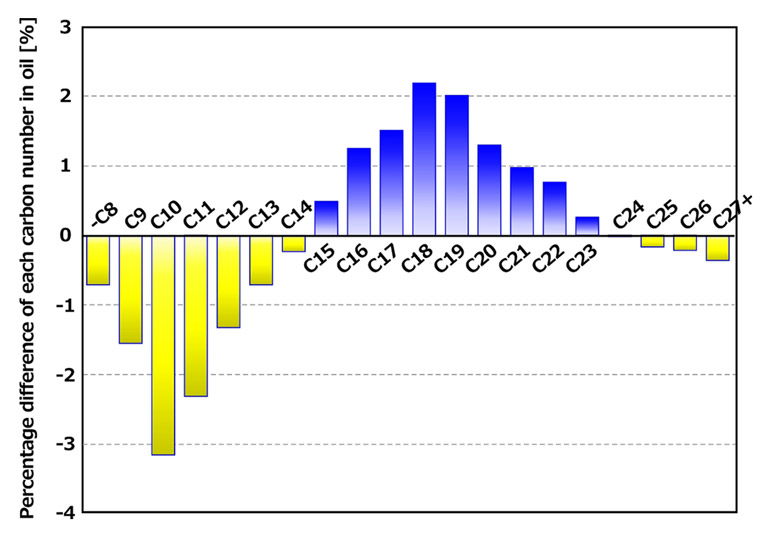
The differences in component ratios are as follows. The carbon numbers in each case differ by within 3.2%, indicating that they are nearly equivalent components.
| Item | Results | Method | |||
|---|---|---|---|---|---|
| Original oil | New oil | ||||
| 1. | Reaction | – | Neutrality | Neutrality | JIS K2252 |
| 2. | Flash point(PMCC) | ℃ | 73.0 | 82.0 | JIS K2265-3 |
| 3. | Kinematic Viscosity(30℃) | mm2/s | 3.479 | 3.710 | JIS K2283 |
| 4. | Pour point | ℃ | -15.0 | -12.5 | JIS K2269 |
| 5. | Residual carbon | Mass% | 0.01 | 0.04 | JIS K2270-2 |
| 6. | Water, KF method | Mass% | 0.0063 | 0.010 | JIS K2275 |
| 7. | Ash content | Mass% | 0.001 | 0.001 | JIS K2272 |
| 8. | Sulphur content | Mass% | 0.0007 | 0.0007 | JIS K2541-6 |
| 9. | Density(15℃) | g/cm3 | 0.8295 | 0.8311 | JIS K2249-1 |
| 10. | Distillation Temperature | JIS K2254 | |||
| 10% distillation temp. | ℃ | 217.0 | 226.0 | ||
| 50% distillation temp. | ℃ | 271.5 | 274.5 | ||
| 90% distillation temp. | ℃ | 326.0 | 328.5 | ||
| 11. | Cetane index | – | 56.2 | 56.9 | JIS K2280-5 |
| 12. | Gross heat of combustion | J/g | 45,990 | 46,010 | JIS K2279 |
| 13. | Cold filter plugging point | ℃ | -10 | -10 | JIS K2288 |
※Please note that the data presented on this page pertains to the prototype stage of the artificial fuel manufacturing unit.

